Chase’s life turned unexpectedly when he was just six years old. As a loving grandmother, I witnessed the remarkable strength and resilience of my grandson as he faced a traumatic brain injury caused by F.I.R.E.S. (Febrile Infection-Related Epilepsy Syndrome). This harrowing journey has not only transformed Chase’s life but has also highlighted the incredible power of hope and the potential of unconventional treatments.
It all began when Chase had just started first grade. Three weeks into the school year, he developed strep throat and was prescribed amoxicillin. However, Chase’s condition worsened, and by day five of treatment, he began experiencing continuous seizures. In a desperate race against time, he was airlifted from a local emergency department to the Pediatric Intensive Care Unit at the Children’s Hospital of Philadelphia (CHOP).
Chase’s life hung in the balance as he was put on life support, administered high doses of antiseizure medications, underwent medically induced hypothermia, and received a ketogenic diet through an IV. The odds were stacked against him, with doctors giving him only a 30% chance of survival, and the prognosis for F.I.R.E.S. was grim. My daughter, a single parent at the time, and I scoured the internet for any glimmer of hope, any treatment that could offer a lifeline to our precious Chase.

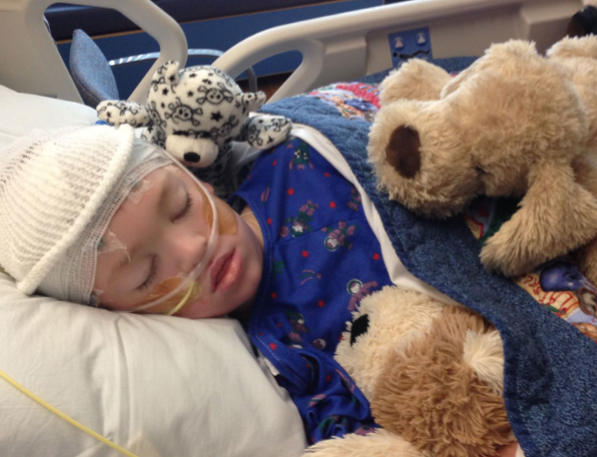
After weeks of relentless research, we stumbled upon a small mention of a clinical trial involving cannabis oil for children with intractable epilepsy, specifically Dravet and Lennox-Gastaut syndromes. We approached CHOP with this information and were granted permission for the “compassionate use program” of Epidiolex, a new medication developed by GW Pharmaceuticals undergoing clinical trials. A compassionate use program, also known as expanded access, provides access to investigational drugs, biologics, and medical devices. These programs are used to treat patients with serious or lifethreatening diseases or conditions for which there are no satisfactory treatment options. The results were nothing short of miraculous; Chase’s seizures began to decrease almost immediately.
With the help of Epidiolex, Chase’s seizures eventually stopped completely. However, the journey was far from over. He was transferred to CHOP’s rehabilitation center, where he had to relearn basic functions, such as swallowing, chewing, standing, sitting, toileting, and speaking. As a former registered nurse, I had witnessed countless medical challenges, but this experience was one of the most emotionally taxing. I had entered the ordeal believing that a top-tier children’s hospital would provide all the answers, but the reality was far more complex.
Today, Chase is 15 years old, a testament to the power of perseverance and medical innovation. While he continues to require a ketogenic diet, several antiseizure medications, and a device implanted in his chest to interrupt breakthrough seizures, Epidiolex remains a vital part of his treatment regimen. The traumatic brain injury he sustained has left him with receptive and expressive aphasia, necessitating his use of a communication board for interaction. He is enrolled in high school special education, defying the odds stacked against him since that fateful day when F.I.R.E.S. left a lasting mark on his life.

Chase’s journey demonstrates the unyielding spirit of children and the power of families who refuse to give up in the face of adversity. It underscores the importance of medical research and compassionate use of emerging treatments and inspires all those facing their battles with rare life-threatening and debilitating conditions. Chase’s story reminds us that even in the darkest times, there is always a glimmer of hope, and with determination, love, and medical breakthroughs we can overcome the greatest challenges.


